Skin Analytics × Scarlet
Bringing a world-first AI medical device to the European market
How Skin Analytics certified a life-saving Class III AIaMD, enabling quicker access to skin cancer diagnosis for hundreds of millions of patients
“If you're looking for a Notified Body that understands SaMD then Scarlet is the one to work with.”
James Hamlyn, QA/RA Director, Skin Analytics
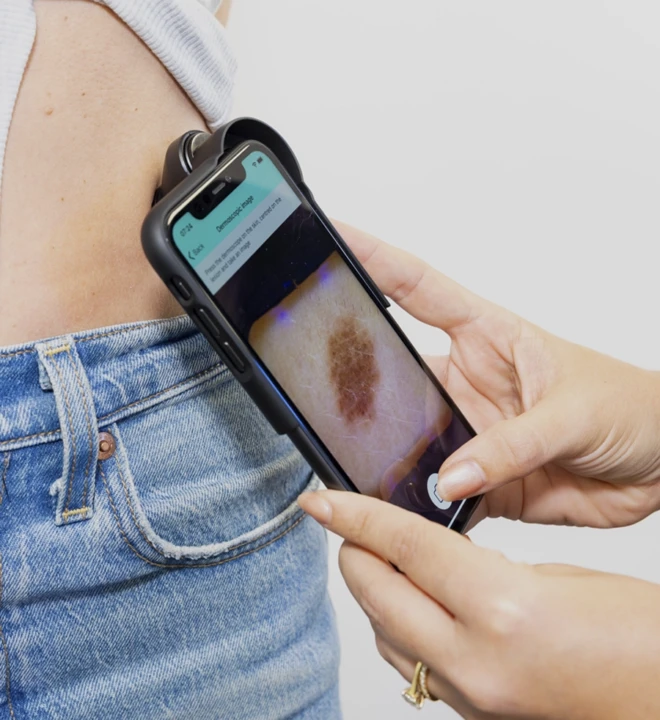
The Challenge
Skin Analytics were looking at a long wait to get their proven skin-cancer-detection product DERM to the European market, and were seeking a Notified Body with AI expertise
Customer Snapshot
- Growing start-up with UK success, looking to expand internationally
- Developed a transformational skin-lesion-analysis SaMD that had already been widely deployed in the NHS
- Seeking swift EU MDR certification from a Notified Body that understands AI
Impact
- 99.8% accurate in ruling out high-risk skin cancer — compared to face-to-face dermatologist evaluations of 98.9% [source]
- DERM became the only AI approved to make clinical decisions autonomously in the cancer space
The Scarlet Process
Scarlet's AI expertise, structured & clear assessment process, and SaMD-friendly technical file submission — led to a highly efficient time to certification for Skin Analytics.
How It Worked
To ensure no unnecessary delays, we:
- Deployed Scarlet's structured certification process, with regular expert dialogue — avoiding ambiguity on progress
- Used Scarlet’s AI specialists to efficiently understand Skin Analytics' product — avoiding needless back and forth
- Ran Scarlet’s SaMD-specialised assessment to streamline submission — avoiding wasted effort
The Results
Scarlet’s approach resulted in:
- A six-month time to EU MDR certification
- Approval of DERM significantly ahead of the expected timeline
- Skin Analytics’ proven technology is now available, ahead of schedule, to patients and healthcare systems across the European market
“We were looking at a long and unclear path to certification. Scarlet’s expertise in AI changed that. They were able to efficiently understand a highly novel AI product. That meant we got to market far quicker than originally expected.”
James Hamlyn, QA/RA Director, Skin Analytics
Need to certify an AI medical device/SaMD?
Schedule a call or demo, or simply get more info about the Scarlet process and platform.
