Lucida Medical × Scarlet
Certifying the latest AI model of a Class IIb medical device in 7 days
How Lucida Medical got a product update approved for their MDR-certified, prostate-cancer-detection system in one week
“We transferred to Scarlet because our mission is to detect cancer as early as possible — we need to be able to update our devices regularly and without friction.
Scarlet’s AI expertise and responsiveness meant that as soon as we completed the certificate transfer, we were able to get a product update approved in 7 days.”
Stefani Aleksandrova, Head of Regulatory Affairs, Lucida Medical
The Challenge
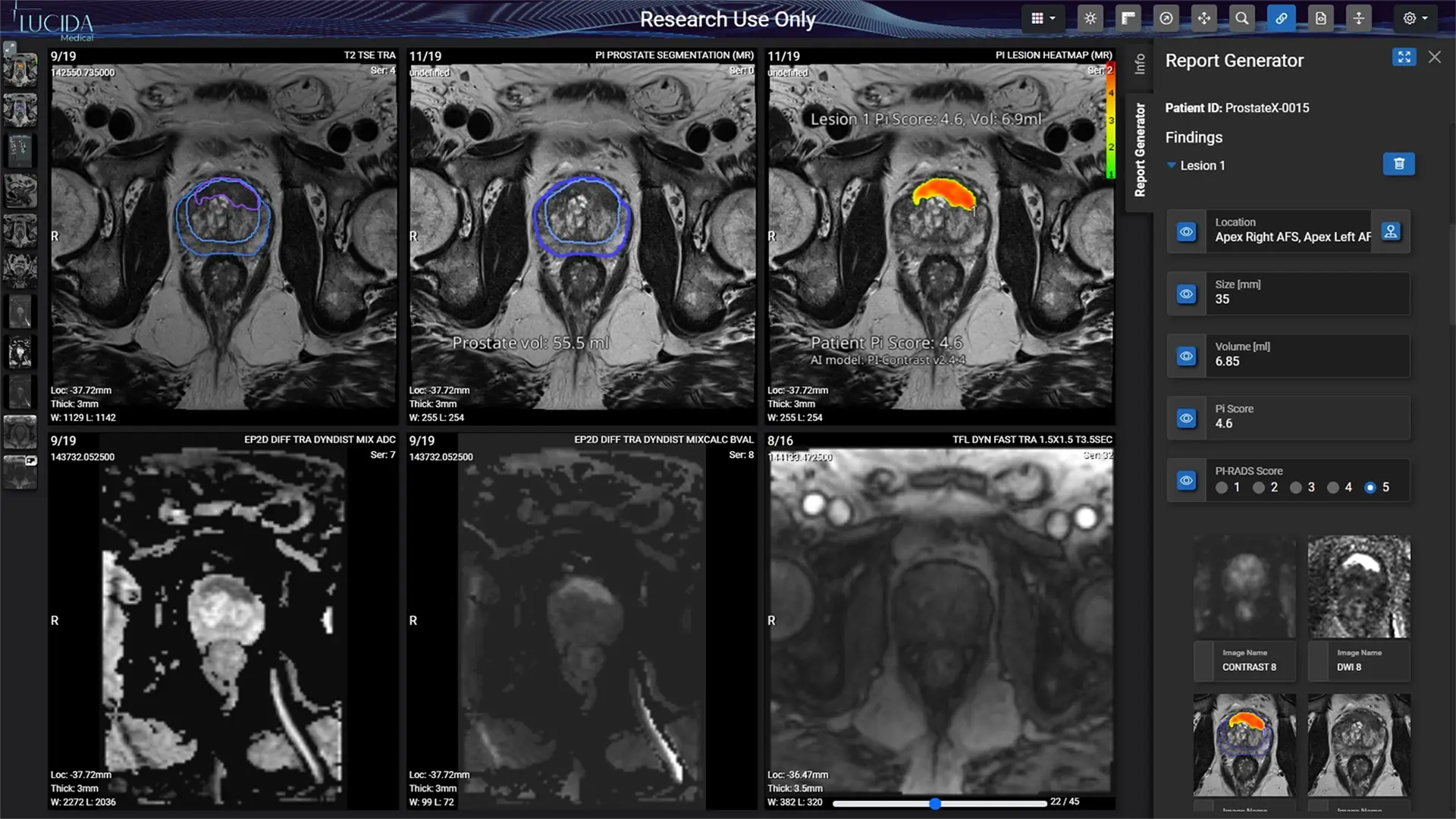
Lucida Medical needed a Notified Body with SaMD expertise that would enable them to consistently get the latest and best version of their ground-breaking AI system Prostate Intelligence (Pi) into the hands of users and to the patients who need it
Customer Snapshot
- Successful start-up rapidly iterating their product
- Developed a transformational prostate-cancer-detection system, that had already been widely deployed in the NHS
- Seeking seamless approvals of changes to their system under EU MDR by using a Notified Body that understands AI
Impact
- Validated in a large clinical study in the UK NHS, PAIR-1
- Detects 95 out of 100 cases of clinically significant prostate cancer, with results confirmed by biopsy

The Scarlet Process
Scarlet's AI expertise, structured transfer and change-review processes, and dedicated support led to a smooth certificate transfer and one-week change approval for Lucida Medical.
How It Worked
To ensure a seamless process, we:
- Used Scarlet’s AI specialists to efficiently understand Lucida Medical’s product - avoiding needless back and forth
- Ran Scarlet’s structured change-request system to streamline processing - avoiding wasted effort
- Maintained regular contact with Lucida Medical to ensure they had full visibility on timelines - avoiding the uncertainty that prevents reliable planning
The Results
Scarlet’s approach resulted in:
- Dual EU MDR & ISO 13485 certificate transfer completed in weeks
- Approval of an update to Prostate Intelligence (Pi) in just seven days
- Lucida Medical’s newest and best version of Pi getting to healthcare institutions and patients across the NHS
“The transfer to Scarlet was seamless.
We received regular updates throughout, the process was clear, and we always knew what was going on.
We had no idea the transfer could be so swift.”
Stefani Aleksandrova, Head of Regulatory Affairs, Lucida Medical
Start your transfer now
Need to certify an AI medical device/SaMD?
Schedule a call or demo, or simply get more info about the Scarlet process and platform.
