From monolithic to modular: How defining a single product as multiple software medical devices can streamline certification
Can having a higher number of devices actually make certification more streamlined than it would be for a single device? Counterintuitively, it can.
Certifying medical devices has always been a balancing act between innovation and regulation. For software products in particular, the instinct is often to define the broadest possible intended purpose — capturing multiple conditions, user groups, and modes of action in a single device.
At first glance, this seems efficient: one device, one file, one pathway through conformity assessment.
But in practice, the "monolithic device" approach often creates more problems than it solves.
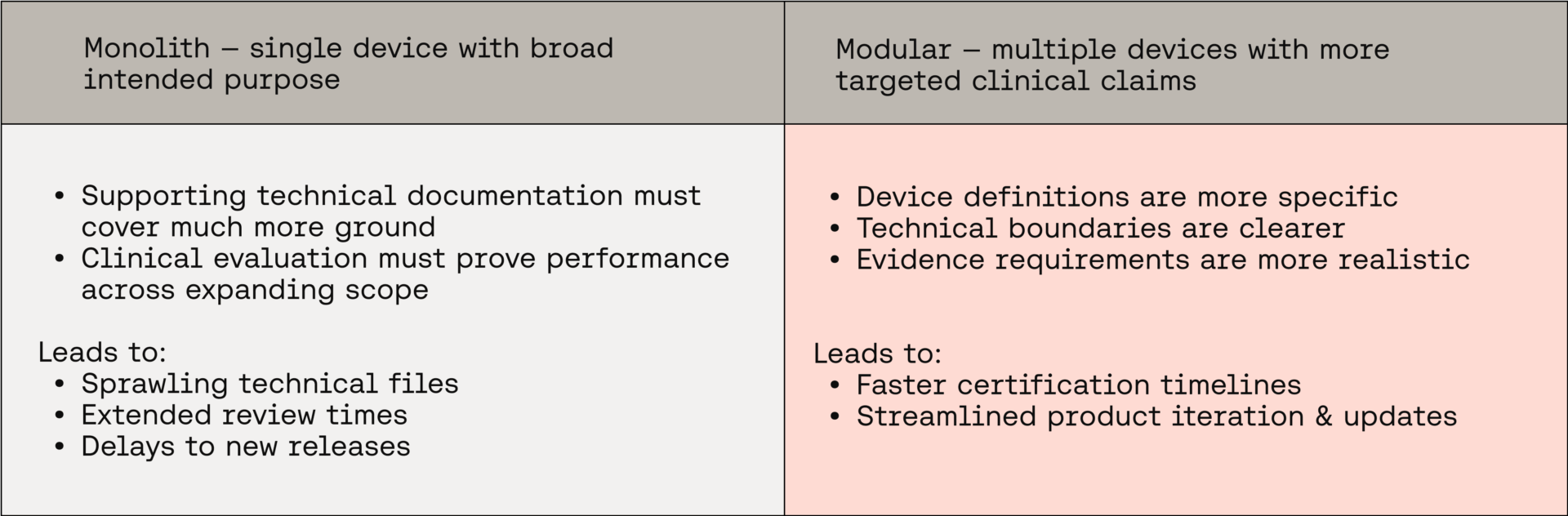
The burden of the monolith
A broad intended purpose means the supporting technical documentation must cover a wide variety of use cases, patient populations, and risk profiles.
Clinical evaluation becomes a bottleneck: it is no longer about generating or identifying evidence for a focused claim, but instead about proving performance across an ever-expanding scope.
RAQA professionals know where this leads: sprawling technical files, extended review times, and significant delays in bringing new features to market. Paradoxically, what was meant to streamline certification often ends up slowing it down.
Rethinking what counts as a "device"
A more pragmatic approach is to start with the product, not the regulation.
Consider each feature in isolation: what does it do, who does it serve, and does it have a medical purpose? From this perspective, it becomes clear that a product may actually be composed of multiple devices.
For example:
- An algorithm that generates triage recommendations for one patient group may constitute a device
- A second algorithm designed for another patient group could be a separate device
- Supporting software modules may instead fall into the category of accessories or non-certifiable features
By acknowledging functional and clinical differences upfront, manufacturers can build device definitions that are more specific, evidence requirements that are more realistic, and documentation that is more manageable.
A modular pathway to compliance
Splitting a product into multiple devices may seem like adding complexity, but for RAQA professionals it often reduces regulatory friction. Narrower device scopes mean more targeted clinical claims and clearer technical boundaries.
Crucially, this modular approach enables incremental development. When new functionality is introduced, RAQAs can determine whether it expands an existing device or defines a new one. If it’s the former, the change can often be handled through a tightly scoped substantial change request. If it’s the latter, a new device can be documented and assessed without destabilising the certification of existing ones.
In practice, this flexibility can shave months off review timelines and provide a more sustainable framework for continuous product evolution.
Beyond efficiency: a strategic shift
Reframing a product as multiple devices is more than a paperwork strategy. It reflects a shift in mindset: away from viewing regulation as an obstacle to be minimised, and towards using regulatory structure as a tool for product clarity.
For those in regulatory affairs the reward is twofold: a stronger foundation for compliance and a clearer pathway for innovation.
Instead of being locked into the complexity of a monolithic device, manufacturers can embrace modularity — building safer, more adaptable technologies that reach patients sooner.

Want Scarlet news in your inbox?
Sign up to receive updates from Scarlet, including our newsletter containing blog posts, sent straight to you by email.