SaMD equivalence under EU/UK MDR: What you need to know
Claiming equivalence for Software as a Medical Device (SaMD) under EU and UK regulations is no easy feat. Manufacturers must demonstrate technical and clinical equivalence, ensuring both devices have similar, or in some cases the same, attributes.
Regulatory scrutiny is strict, particularly regarding algorithm transparency, cybersecurity risks, and data access. This makes it difficult to justify equivalence without comprehensive documentation, scientific justification, and substantial insight into the equivalent software. MDCG 2020-5 and MEDDEV 2.7/1 Rev 4 provide guidance on how to tackle this for EU and UK markets respectively.
What's the benefit of claiming equivalence?
Claiming equivalence can provide different ways to supply the clinical data required for your device. If there is an appropriate equivalence claim, the data from the equivalent device can be used to support the clinical evaluation (EU MDR Annex XIV Part A (1); MDCG 2020-5, Section 2.3).
Under EU MDR, clinical data can originate from:
- clinical investigation(s) of the device concerned
- clinical investigation(s) or other studies reported in scientific literature, of a device for which equivalence to the device in question can be demonstrated
- reports published in peer reviewed scientific literature on other clinical experience of either the device in question or a device for which equivalence to the device in question can be demonstrated
- clinically relevant information coming from post-market surveillance, in particular the post-market clinical follow-up (PMCF)
Claiming equivalence can potentially eliminate the need for additional clinical trials, accelerating market access, and reducing development costs.
Which characteristics need to be analysed between devices?
In order to make a successful equivalence claim, both EU and UK regulation require similarity in technical and clinical characteristics (biological characteristics are generally not relevant to SaMDs). While these are mostly consistent between regulatory markets, there is one technical-characteristic difference (see ‘Conditions of use’ below):
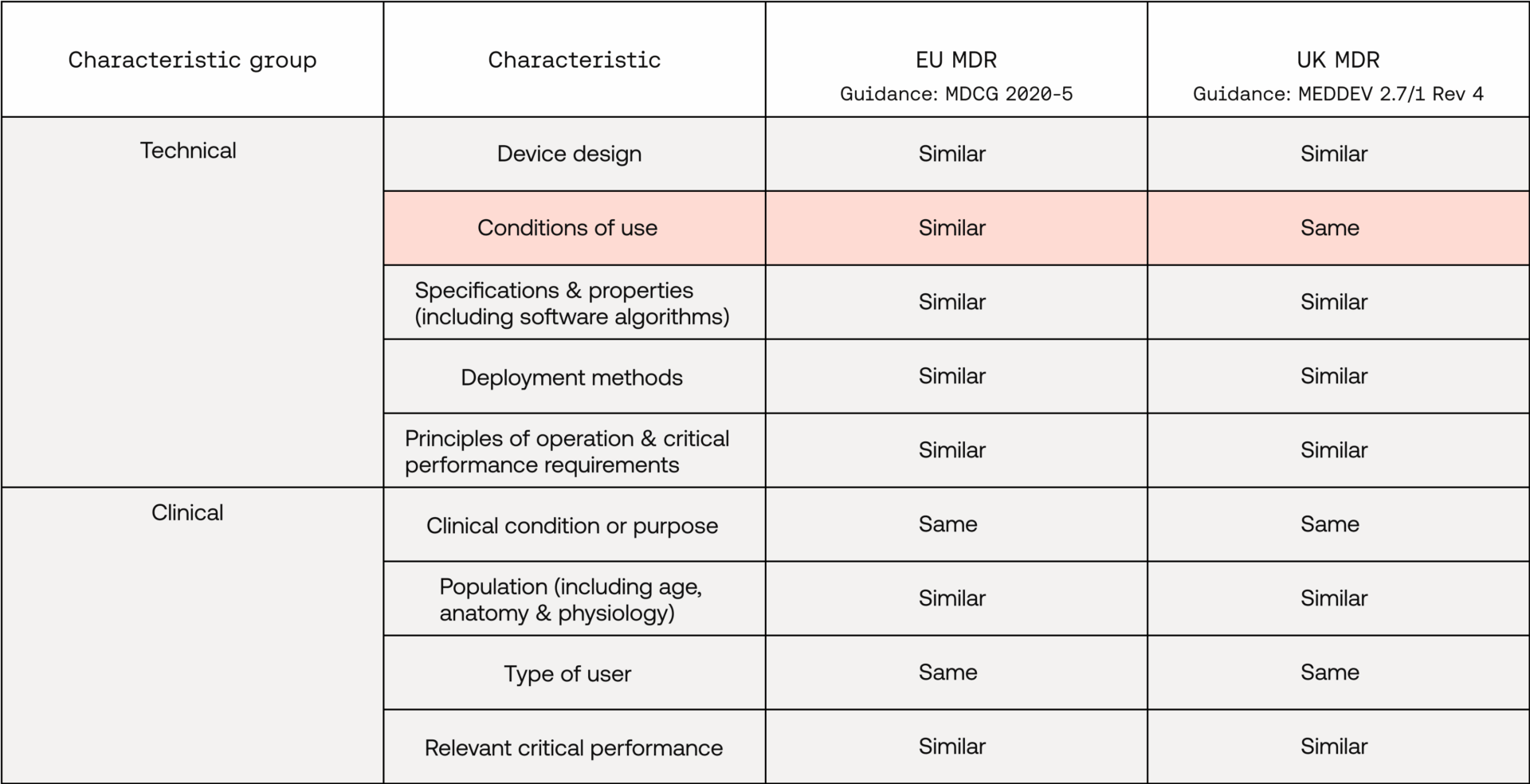
How do you analyse these characteristics?
“considerations of equivalence shall be based on proper scientific justification”
MDR, Annex XIV Part A (3)
To make a successful equivalence claim, you need to provide scientific evidence that supports that there are no differences between your device and the equivalent device which could significantly impact performance or safety. For some characteristic analyses, comparing software algorithm descriptions, data inputs and outputs, technical performance test reports, and clinical performance outcomes (e.g. sensitivity and specificity) could be used to provide scientific justification. Similarly, for other characteristic analyses where it may be difficult to provide proper scientific justification, e.g. for the device design characteristic, a thorough comparison of key technical documentation (e.g. Instructions for Use) may provide sufficient evidence. MDCG 2020-5 also recommends using a gap analysis to evaluate any clinically significant differences between devices.
Similarly, when claiming equivalence, literature searches for published and non-published sources regarding the proposed equivalent device are required (MEDDEV 2.7/1 Rev. 4, Section 4).
Frequently Asked Questions
The above points raise questions. So let's look at some that come up often.
Can I claim equivalence to a non-CE-marked device?
Yes, but under strict conditions:
- You have full access to technical and clinical data
- Clinical investigations for the equivalent device followed international guidelines
- Data is sufficient to meet EU MDR requirements for safety and performance
This is most feasible when the equivalent device is:
- From the same manufacturer
- Proven to have similar deployment and use conditions
Can I claim equivalence to part of a device?
Yes, but only under exceptional conditions. Equivalence to a stand-alone component (e.g. a software module) is allowed if (MDCG 2020-5, Section 3.2):
- The device is from the same manufacturer
- The component functions independently from other features
- Clinical and technical characteristics are similar
- Interactions between systems do not affect safety or performance
Can I claim equivalence to a device from a different manufacturer?
This is significantly more complex. The EU MDR (Annex XIV, Part A, Section 3) and MDCG 2020-5 stress that this is only possible when the manufacturer can demonstrate access to sufficient levels of detail about the equivalent device’s technical and clinical characteristics. MDCG 2023-7 provides additional guidance about the sufficient levels of access to data required for an EU equivalence claim.
To do so, you must provide:
- Documented proof of technical and clinical equivalence (e.g. specifications, software architecture, performance metrics)
- Evidence of access to data through formal agreements (e.g. contractual access, data-sharing arrangements, co-marketing agreements)
- A scientific justification showing that any differences between devices do not impact the safety or clinical performance of your device
Without full access to the required data, equivalence claims to a third-party device will not be accepted under the EU MDR (MDCG 2020-5, Section 3.1).
Key challenge: Unlike with your own device, competitors are unlikely to provide confidential technical files or clinical datasets. In such cases, consider using the device as a similar device rather than claiming equivalence.
An example equivalence-claim scenario
To bring the above into sharper focus, let's look at a hypothetical example.
Company 1 has a CE-marked SaMD that predicts stroke risk based on blood pressure data. Company 2 has developed a device that predicts stroke risk based on blood pressure and cholesterol-levels prediction, and has a similar intended purpose, clinical condition, user profile, and patient population. Company 2 is seeking initial EU MDR certification, claiming equivalence to Company 1's device.
| Parameter | Device Under Evaluation | Proposed Equivalent Device |
| Manufacturer | Company 2 | Company 1 |
| Intended purpose | Stroke risk prediction & cholesterol estimation | Stroke risk prediction |
| Patient population | Adults: 45-80 years | Adults: 30-75 years |
| User | Healthcare professionals | Healthcare professionals |
| Deployment | Hospital EHR integration | Hospital EHR integration |
| Clinical performance | 92% accuracy, sensitivity/specificity balanced | 91% accuracy, similar balance |
| Technical performance | Real-time prediction with cloud sync | Real-time prediction |
| Software algorithm | Blood pressure and lipid-count data input | Blood pressure data input |
- As the devices have different manufacturers, access to key documentation such as instructions for use, software algorithm descriptions, validation studies, and risk management is required (likely through a data-sharing agreement)
- Although the algorithm has additional data input and outputs, the principle of operation and clinical performance remain consistent
- A gap analysis can be conducted to assess the impact of the new input parameter (cholesterol) and demonstrate no clinically significant differences
Conclusion: A valid equivalence claim is likely possible, leveraging existing clinical data and supporting documentation, provided any new performance characteristics are addressed with scientific justification.
If equivalence cannot be claimed
Even if an equivalence claim isn’t possible, data from similar devices can still provide support. In particular they can help with:
- Establishing the clinical state of the art
- Risk-management justifications
- Context for your device’s safety profile
MDCG 2020-5 acknowledges the value of similar-device data for clinical-evaluation planning and risk-assessment purposes.
Summary
Claiming equivalence for SaMD is a strategic way to reduce development time, costs, and evidence-generation requirements. But it’s only viable with rigorous comparative documentation, transparent scientific justification, and often, access to proprietary information.

Want Scarlet news in your inbox?
Sign up to receive updates from Scarlet, including our newsletter containing blog posts, sent straight to you by email.


