How to determine the device classification of SaMD under EU MDR
Determining the appropriate device classification for Software as a Medical Device (SaMD) under the EU’s regulatory framework is crucial for manufacturers aiming for regulatory approval and market access.
Higher-risk software medical devices require robust evidence to demonstrate safety and performance. EU MDR Annex VIII, Rule 11 outlines the classification rules for software. Applying the clauses of Rule 11 can be challenging, for example trying to ascertain what constitutes “serious deterioration or immediate patient danger”.
MDCG 2019-11 rev.1, MDCG 2021-24, and IMDRF SaMD WG-N12Final:2014 offer guidance on applying Rule 11. MDCG guidance provides more detail on the classification of SaMD and additional considerations for applicable rules from EU MDR, Annex VIII.
The IMDRF framework proposes four SaMD categories (I-IV) that help manufacturers systematically evaluate their software’s clinical risk, particularly when used in conjunction with the risk matrix provided by MDCG 2019-11 rev.1.
It’s important to demonstrate how these frameworks and rules apply for the device with a clear, well-documented rationale to support the proposed device class.
Understanding the clinical context
In what is an increasingly complex clinical-use environment, there are many situations affecting risk for patients. In order to successfully determine the correct classification of your device, understanding and clearly defining the intended purpose and clinical workflow and applying these to the relevant EU MDR Annex VIII rules and device-classification guidance is crucial.
For example, when applying the IMDRF framework definitions, consider an AI-based software that provides clinical management guidelines for abdominal pathology. The software’s scope of abdominal pathology may affect its classification. IMDRF states that for a device to inform clinical management, “the SaMD will not trigger an immediate or near-term action”. If the device scope includes time-critical, life-threatening abdominal conditions, it is difficult to justify that it will NOT trigger an immediate or near-term action.
It is equally important to consider the intended users of the device and the intended use environment. Devices used by lay users in a "serious situation or condition" without support from specialised professionals are escalated to "critical" in IMDRF. This illustrates the importance of all aspects of the intended purpose and clinical workflow. Devices used without support from healthcare professionals, or used in environments where more severe conditions are likely to be encountered such as Critical Care, may indicate a more severe healthcare situation.
The intended purpose, including intended conditions, target population, users, use environment, and features of a device should depict an aligned clinical narrative to avoid contradictions in the classification.
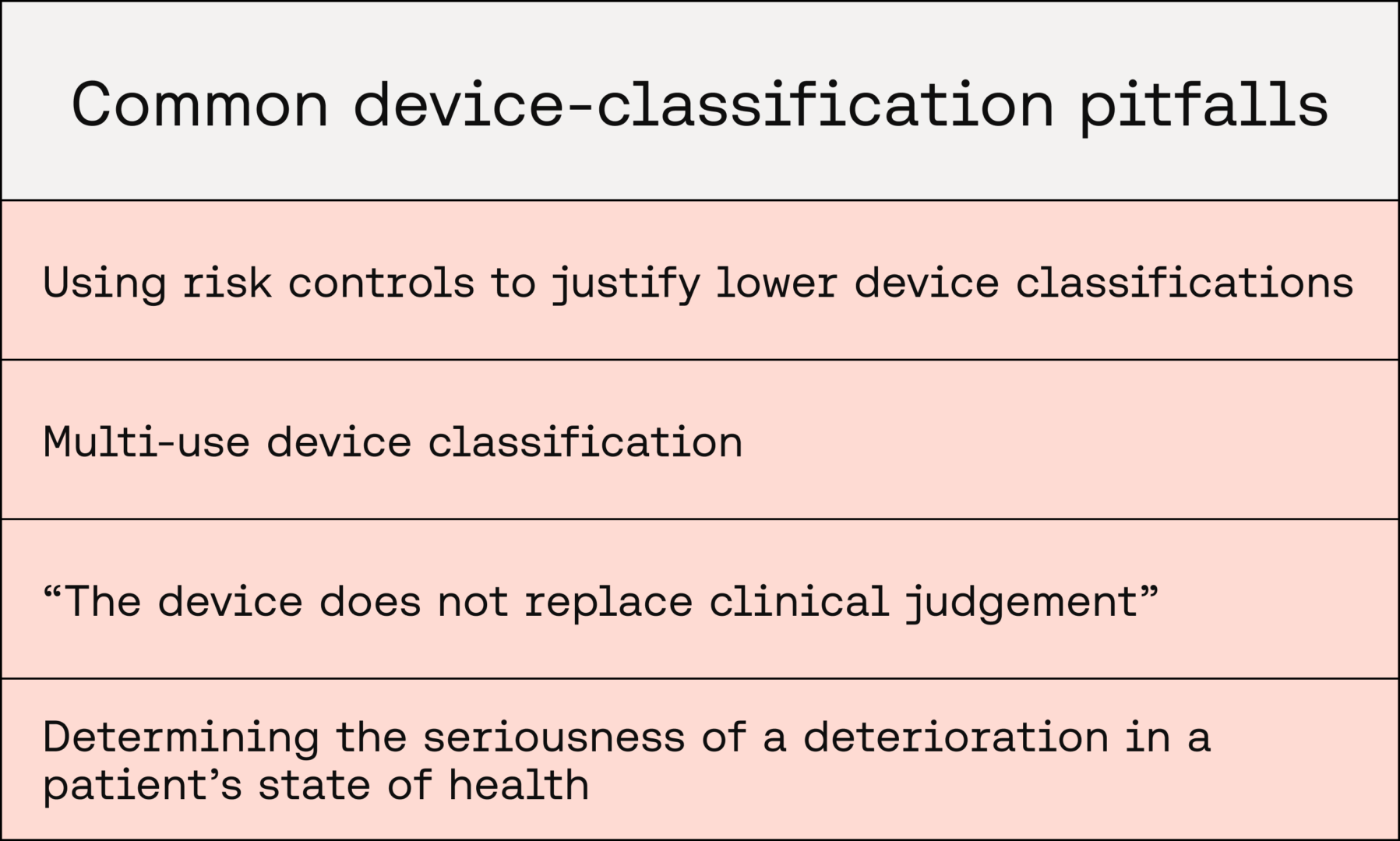
Common pitfalls
-
Using risk controls to justify lower device classifications
While risk controls are important in device development for minimising patient-safety risks, they do not influence the category of SaMD. The classification should be solely based on the intended purpose to justify the significance of information provided by the SaMD and the state of the healthcare situation. As such, downstream risk controls, including safe design, protective measures, and information for safety, should be avoided in the classification rationale.
-
Multi-use device classification
Devices capable of being used across multiple clinical conditions, users, and use environments are becoming increasingly common. When a device can be used across multiple circumstances, it should be categorised by the most severe case that applies based on the intended purpose and description of use (IMDRF 7.1).
A device that has the general population as its intended target population would include vulnerable populations such as paediatric patients, and thus would entail higher potential risks. So the most severe conditions should be described in the classification rationale, unless such populations are clearly excluded in the intended target population.
-
“The device does not replace clinical judgement”
Manufacturers may argue that their device “does not replace clinical judgement” as a justification for a lower-severity healthcare situation or condition, however this is not considered sufficient evidence to think the healthcare situation or condition is mitigated. Their severity takes into account the intended conditions, users, use environment, and target population.
It’s also important to consider how the device affects clinical workflow and how it might introduce bias into clinical judgement. The device not replacing clinical judgement solely implies that the significance of the information provided by the SaMD at most drives clinical management.
-
Determining the seriousness of a deterioration in a patient’s state of health
Within healthcare, there are a multitude of situations and complications that may result in a serious deterioration or death. Importantly, the likelihood of these situations is relevant for determining the severity of the healthcare situation.
MDCG 2019-11 rev.1 clarifies that whether or not “such decisions, if based on incorrect information from the MDSW (Medical Device Software), are reasonably likely to have an impact” (on patient health) should be the basis for determining a higher risk class.
So if the device fails to diagnose an indolent condition with subsequent prognostic implications being non-serious, it is reasonable to think that it’s a non-serious situation or condition.
The bottom line is to consider the most reasonably likely worst-case scenario when thinking about risk and classification.
Key takeaways
- Clearly defining and documenting the intended purpose, clinical workflow, and clinical context are essential for accurate classification
- Risk controls and justifications such as clinical judgement are not considered adequate for explaining lower device classifications; classification must objectively reflect the significance of the information and healthcare situation of the intended SaMD use
- If the device serves multiple purposes or populations, classification must align with the highest-risk intended scenario, condition, or population described in its intended use
In an evolving regulatory landscape, achieving the correct SaMD classification is not just a procedural step, it’s a decision grounded in clarity of purpose, clinical understanding, and robust justification. Getting it right from the outset ensures not only compliance, but trust, credibility, and access to the patients who need it.
Recommended Reading

Want Scarlet news in your inbox?
Sign up to receive updates from Scarlet, including our newsletter containing blog posts, sent straight to you by email.