Search and rescue: Save yourself from the most common SaMD literature search and review mistakes
Thoroughness and accuracy are key when preparing a literature search and review for regulatory submission, but even experienced teams can make critical mistakes that compromise the quality of their submission.
Whether it’s poorly structured search terms or insufficient documentation, such errors can delay approval of your device. Our recent guide took you through seven steps for a proper literature search and review, in this post we’ll explore some of the most common mistakes and how you can avoid them.
Non-comprehensive search of scientific literature databases
MEDDEV 2.7/1 rev 4 A4 calls for a comprehensive search strategy, normally involving multiple databases. Database selection is important for comprehensiveness and should be tailored to, and justified for, the domain, device, or research question.
Non-comprehensive internet search strategy
An internet search should complement database searches and be more than a simple Google search. Internet sources may include manufacturer websites or websites for professional bodies. Additionally, European Competent Authorities, the U.S. Food and Drug Administration (FDA), and possibly others, publish field safety corrective actions and other data that may be relevant to searches.
One option is to use Google’s Advanced Search options to refine your searches and find specific information on websites that lack their own search functionality.
Poorly structured search terms
While Boolean logic and filters can help narrow the search to identify the most relevant studies and minimise retrieval of irrelevant ones, poorly structured search terms can exclude important studies. On the other hand, an overly broad search is not a problem from a regulatory perspective, but it will lead to more work than necessary for your team.
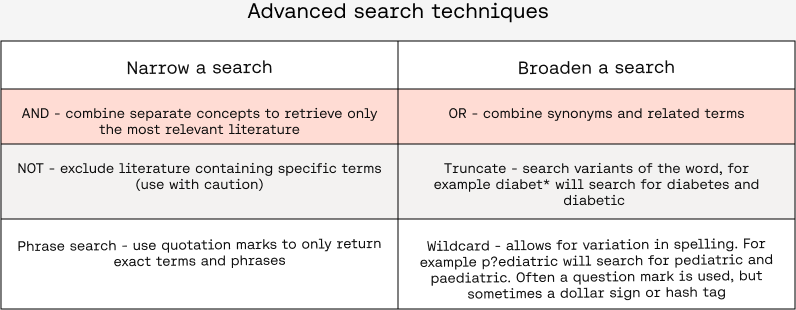
Boolean logic
Boolean operators can narrow (AND, NOT, phrase search - “”) or broaden (OR, truncate - *, wildcard - ?) your search, but use these with caution in order to not accidentally exclude relevant literature.
NOT
It might be tempting to use NOT to exclude literature, for example, to exclude literature on animals or children in devices meant for adults. However, using NOT may unintentionally exclude relevant literature as some literature might be relevant to both animals and humans, or both adults and children.
AND versus OR
Carefully consider which concepts should be combined with AND versus which concepts should be combined with OR. For example, when trying to identify literature to determine the state of the art, using AND with terms such as software or AI might overly limit the search to not include current standard practice (if standard practice isn’t using software/AI).
Generally, you can use OR to capture synonyms and related terms for the same concept (e.g. AI OR artificial intelligence) while you can use AND to combine all the distinct concepts related to your search [e.g. (all terms related to AI) AND (all terms related to diabetic retinopathy) AND (all terms related to screening)]
Note that constructing complex search queries varies between databases. Always check the documentation of each database on how to correctly build these queries.
Insufficient detail for reproducibility
Database and internet searches need to be documented with exact search terms and methods such that they can be reproduced. Ensure boolean operators (e.g. AND, OR) and filters (e.g. date, publication type) are also captured. You can ensure the exact terms are included by copying your search directly from the database.
Failure to search citations of returned literature
MEDDEV 2.7/1 rev 4 Annex 4 states:
- "Citations referenced in scientific literature can be important and should be screened. Literature found to be relevant is likely to cite other literature that is of direct interest to the manufacturer."
Don't forget to include this as a step in your search strategy.
Lack of, or poor, justification for decisions
As there are many acceptable ways a literature search and review can be carried out, justification of all decisions is important. In fact, MEDDEV 2.7/1 rev 4 includes the word(s) justif* 60 times! Justifications for all choices should be clear and reasonable.
A search that isn't recent
Literature searches should be recent, including literature published near the time of submission. Justifying a search that is more than six months old may be difficult.
Conclusion
A well-executed literature search and review can make or break your regulatory submission. By avoiding common mistakes such as non-comprehensive searches or inadequate justification of decisions, you’ll build a proper case for the safety and effectiveness of your medical device.

Want Scarlet news in your inbox?
Sign up to receive updates from Scarlet, including our newsletter containing blog posts, sent straight to you by email.